Additional Claims Filing Details for the Flu Vaccine When filing a claim use one of the following CPT codes. 90662 90674 90682 90686 90688 90756 or G0008.

Flu Vaccine Dosing And Coding Gsk Flu
Influenza virus vaccine quadrivalent IIV4 split virus preservative free for intradermal use.
Cpt code for flu vaccine visit. Billing Instructions by Claim Type For professional services billed on a CMS 1500 837 P claim form bill the Vaccine Toxoid CPT Code along with its correlating administration code 90471-90474. On this page view the below information. Administration Diagnosis Codes.
Reimburse CPT Code 90658 Influenza Virus Vaccine Split Virus for flu shots. If you administer the intranasal influenza vaccine only report 90473. The following CPT codes must be used when billing Medicaid for the 800 administration fee for each immunization administered to Medicaid children.
CMS has established five separate influenza vaccine HCPCS codes to distinguish between the brand-names of influenza vaccines for governmental tracking purposes. Influenza vaccine inactivated IIV subunit adjuvanted for intramuscular use. Codes 90471 and 90472 are reported for flu vaccine injections when no physician counseling is provided at the time of vaccine administration or when vaccines are administered to persons older than.
O Medically appropriate history andor examination o Choose your reporting pathway. Influenza virus vaccine trivalent IIV3 split virus preservative-free for intradermal use. Administration of one injectable vaccine is billed with CPT code 90471 one unit with the EP modifier.
Deletion of code 99201 and revision of codes 99202-99215 o Codes 99201 and 99202 currently both require straightforward MDM Addition of a shorter 15-minute prolonged service code 99XXX Components for code selection. If you administer an injection of the influenza vaccine only report 90471. Only receive VFC vaccine at a Federally Qualified Health Center FQHC or Rural Health Center RHC.
Do not report G codes in conjunction with CPT codes. Influenza vaccine inactivated IIV subunit adjuvanted for intramuscular use FDA approved for adults 65 years of age and older 90654. Replace the code above with the correct vaccine code below.
Influenza virus vaccine quadrivalent IIV4 split virus preservative free for intradermal use. Make sure to use these new codes in your medical billing. Find the right HCPCS CPT and ICD-10 codes for the flu shot.
CPT codes covered if selection criteria are met. Fluzone High Dose IMInfluenza High Dose 65 yrs 05ml PFS Sanofi Pasteur PMC AKA. The code you use is dependent on the manufacturer of the vaccine.
If you administer the intranasal influenza vaccine only report 90473. Q2034-Q2039 Use the proper CPT code based on the vaccine type administered. If both vaccines are administered on the same day providers are entitled to receive payment for both administration fees.
09112013 V5138 Vaccine trade name or common name Best WAIIS Selection State Supplied Age Range Dose Route Manufacturer NDC Number CPT code CVX code Influenza Conti. Submit claims within 30 days to WPS Military and Veterans Health WPS MVH TriWests claims processor. Do not use CPT code 90653.
People with Medicare pay nothing for a flu shot if the doctor or other qualified health provider accepts assignment for giving the shot. 28 rows HCPCSCPT Codes. Vaccine Codes Descriptors.
The appropriate number of units must be billed for each additional immunization administration CPT procedure code with the total charge for all units reflected on the detail. Each additional vaccine single or combination vaccinetoxoid List separately in addition to 9046090471 or 90473 Other considerations to keep in mind when reporting codes 90471-90474. The diagnosis code used for these vaccines and administration is Z23 encounter for immunization.
If you administer an influenza vaccine in addition to other vaccines report the influenza injection with 90472 or the intranasal with 90474. Get more information on facility and bill types. Vaccine CPT Code to Report Administrative CPT Code s to Bill 90472 will only be used if another vaccine is given in addition to the flu vaccine.
For state-supplied vaccine bill the CPT code. Additional injectable immunization administrations are billed with CPT code 90472 with the EP modifier. Q203xInfluenza virus vaccine split virus when administered to individuals 3 years of age and older for intramuscular use Keep in mind that there are several codes for the flu vaccine when you bill for Medicare flu shots.
90630 90653-90658 90660-90662 90664 90672 90673 90674 90682 90685-90688 90756. 90620 Meningococcal B vaccine Bexsero 90621 Meningococcal B vaccine Trumenba. In addition to the long descriptors short and medium descriptors for CPT codes 87636 87637 87426 and 87811 can be accessed on the AMA website along with several other recent modifications to the CPT code set that have helped streamline the public health response to the SAR-CoV-2 virus and the COVID-19 disease.
If you administer an injection of the influenza vaccine only report 90471. Complete List of Vaccine Names and CPTCVX Codes 68 Updated. Get the most up to date list of billing codes payment allowances and effective dates for the 20202021 flu season.
The European Academy of Allergy and Clinical Immunology EAACI has stated that unless there is a history of allergic reactions to any component of the COVID-19 vaccines there. As with other vaccines people who receive the Covid-19 vaccine may experience injection site pain and swelling fever headache fatigue.
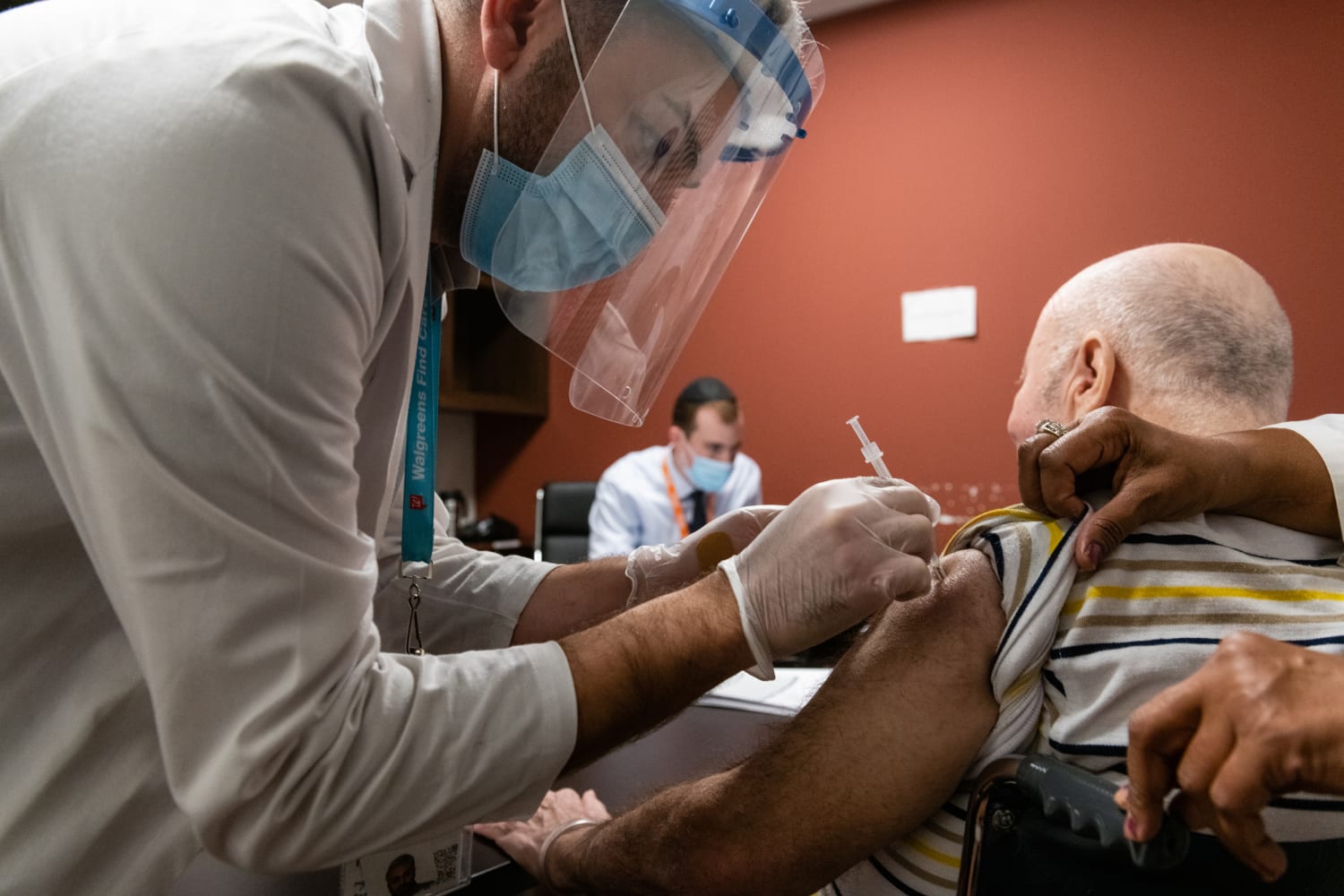
More Allergic Reactions To Covid Vaccine Reported But Overall Remain Rare
If you have had an immediate allergic reactioneven if it was not severeto a vaccine or injectable therapy for another disease ask your doctor if you should get a COVID-19 vaccine.
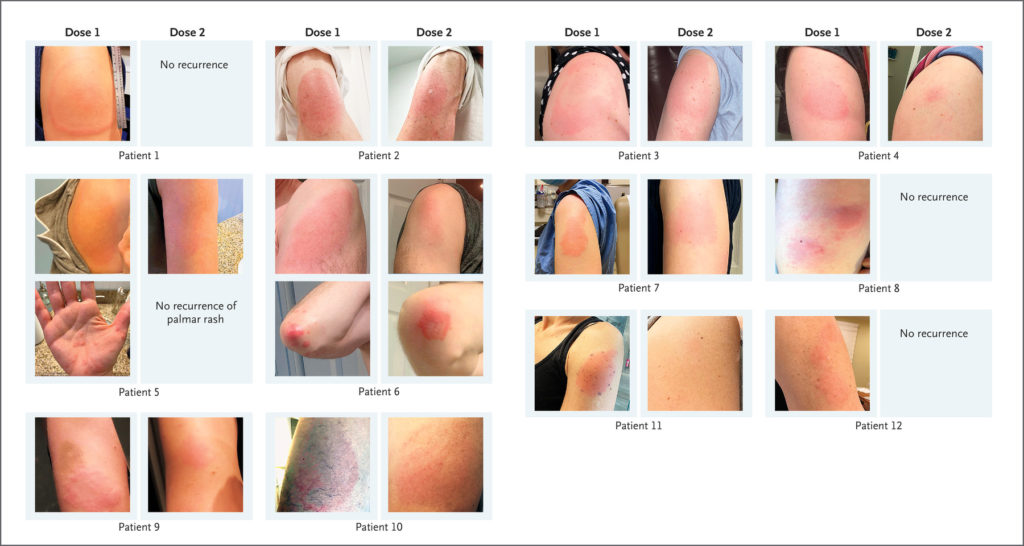
Allergy to covid vaccine reddit. If You Are Allergic to Other Types of Vaccines. Can people with allergies get the COVID-19 vaccine. As the Medicines and Healthcare products Regulatory Agency MHRA has continued their close surveillance of the vaccine roll out they have now advised that individuals with a history of allergy or anaphylaxis to any food can receive any COVID-19 vaccine as long as they are not known to be allergic to any component excipient of the vaccine.
Medical protocols are in place at all Covid-19 vaccination. There is no evidence that people with immune system disorders including allergy immunodeficiencies and autoimmune conditions are at any greater risk of COVID-19 vaccine allergy than the general population. People with a severe allergic reaction anaphylaxis to any component of either an mRNA vaccine or the Johnson Johnson COVID-19 vaccine should NOT receive that vaccine.
The full list of ingredients for the Pfizer vaccine is. Adverse allergic reactions due to the administration of the vaccines developed for the protection of coronavirus disease 2019 COVID-19 have been reported since the initiation of the vaccination campaigns. New unregistered vaccines offer options to those allergic to mRNA Covid-19 vaccines.
Its believed that COVID-19-related allergic reactions are related to polyethylene glycol PEG an ingredient in the vaccines says Dr. US will likely issue allergy warning over Pfizer Covid-19 vaccine. Because the ingredients have not been proven to be causes of Covid-19 vaccine allergic reactions having a negative allergy test to polyethylene glycol or.
Allergy to COVID-19 vaccines. Most people with severe allergies will not react to a COVID-19 vaccination. Here is a breakdown of the three COVID vaccines and their ingredients.
Mar 22 2021 541 PM. Patients Allergic to COVID Vax Got Dose 2 With Graded Dosing Hypersensitivity reactions not a dealbreaker for second dose by Molly Walker Associate Editor MedPage Today. Your doctor will help you decide if it is safe for you to get vaccinated.
36 members in the covidmisc community. MRNA lipids 4-hydroxybutylazanediylbis hexane-61-diylbis 2-hexyldecanoate 2. If you had a severe allergic reactionalso known as anaphylaxisafter getting the first shot of a COVID-19 vaccine CDC recommends that you not get a second shot of that vaccine.
Dr Janil said. If the reaction was after an mRNA COVID-19 vaccine either Pfizer-BioNTech or Moderna you should not get a second shot of either of these vaccines. Yes in most cases with 2 exceptions.
None of the currently approved UK COVID-19 vaccines. Whether some of the reactions are occurring. It will be updated when new information is available.
COVID-19 vaccine side effects indicate the start of an immune response not an allergic reaction. Allergic reactions and people with allergic conditions. We refer to Dr Lai Chan Sees letter Skin rash appeared after first Covid-19 vaccine dose can I go for the second jab June 28.
Expert More than 30000 people in Singapore are not able to take the mRNA vaccines. Statements and guidelines regarding allergy to COVID-19 vaccines. Health Canada is warning individuals with allergies to any of the ingredients in Pfizer-BioNTech COVID-19 vaccine to not receive the shot.
Polyethylene glycol-2000-NN-ditetradecylacetamide 12-Distearoyl-sn-glycero-3-. Francine Orr Los Angeles Times via Getty Images. Covid Alt - towards an alternative adaptive approach to public policy for pandemics.
Some people will get mild short-term side effects from vaccination including injection site reactions fever joint pain muscle aches fatigue headaches or. Ask your doctor if you can get an mRNA COVID-19 vaccine. Messenger ribonucleic acid mRNA lipids SM-102 polyethylene glycol PEG 2000 dimyristoyl glycerol DMG cholesterol and 12-distearoyl-sn-glycero-3-phosphocholine DSPC.
Had a severe allergic reaction after a previous dose of this vaccine had a severe allergic reaction to any ingredient of this vaccine The Moderna COVID-19 Vaccine contains the following ingredients. If You Have a Severe Allergic Reaction to a COVID-19 Vaccine. Official Data showed four people out of about 19000 in the vaccine arm of the trial developed a form of facial paralysis called.
Clinical trial results show Novavax vaccine is safe and prevents COVID-19 Results from a Phase 3 clinical trial enrolling 29960 adult volunteers in the United States and Mexico show that the investigational vaccine known as NVX-CoV2373 demonstrated 904 efficacy in preventing symptomatic COVID-19 disease. Lower risk of side effects may encourage vaccination among lower-income individuals who cant afford to miss a days worth of pay from work.

2nd Big Covid 19 Vaccine Trial Paused Because Of Possible Serious Side Effect Here S What That Means Cbc News
Results from a mid-stage trial showed a strong.

Covid vaccine trial results side effects. Severe systemic adverse events were reported by 5 to 10 percent of trial subjects. Anaphylaxis a severe allergic reaction. Systemic adverse events such as fatigue muscle aches headache and chills are common.
GlaxoSmithKline has unveiled positive interim results from mid-stage trials of a Covid-19 vaccine it is. Side effects are a big barrier for COVID. Allergic reactions severe and otherwise have been reported as possible side effects of the vaccine as a response to certain ingredients in the vaccine.
This included an in-depth review of 19 cases of myocarditis in. Professor Linda-Gail Bekker the co-lead investigator of the Sisonke study previously told Health24 that the vast majority of side effects from the JJ jab have been mild and short-lived a point reiterated in the recently published report. A new real-world study finds fewer side effects after vaccination with the PfizerBioNTech and the AstraZenecaOxford COVID-19 vaccines than reported in phase 3 clinical trials while another paper notes some instances of facial paralysis after receipt of the Pfizer or Moderna vaccine.
In Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials 2 Ron Brown PhD. Still has the potential to. You may have some side effects which are normal signs that your body is building protection.
An international study of COVID-19 vaccine. Calculates the absolute risk reduction for Pfizers and Modernas injections based on their own clinical trial data so that they can be compared to the relative risk reduction reported by these companies. In reaching its conclusion PRAC took into consideration all currently available evidence on both mRNA COVID-19 vaccines ie.
Based on Novavaxs phase 3 clinical trials their COVID-19 vaccine appears to have a substantially lower rate of side effects than the Pfizer-BioNTech or Moderna vaccines. These side effects may affect your ability to do daily activities but they should go away in a. The only evidence on safety of mRNA vaccines comes from small phase I and phase II trials of SARS-CoV-2 vaccines with follow-up typically less than two months.
Approving additional COVID-19 vaccines in the US. Heres a summary of his findings. According to the data the majority 81 of side effects among participants were mild to moderate.
AstraZeneca trial in children shows risk of 15 side effects full list CORONAVIRUS research - funded by AstraZeneca and the National Institute of Health Research -. COVID-19 vaccination will help protect you from getting COVID-19. Many of the secondary outcome measures involve examining or measuring samples from participants up.
Suspected side effects that have been reported by healthcare professionals or patients spontaneously. A volunteer in an earlier phase of the AstraZeneca trial experienced a similar side effect but investigators discovered she had multiple sclerosis that was unrelated to the vaccination according. For the Pfizer vaccine the primary outcomes include reactions like pain at the injection site systemic side effects like a fever or vomiting and adverse events.
But when the Maryland-based biotech firm Novavax announced its latest stunning trial results last week.
The Guidance aims to support WHO. Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA.
Tracking Covid 19 Vaccines And Therapeutics Mckinsey
But its not vaccines that will stop the pandemic its vaccination.
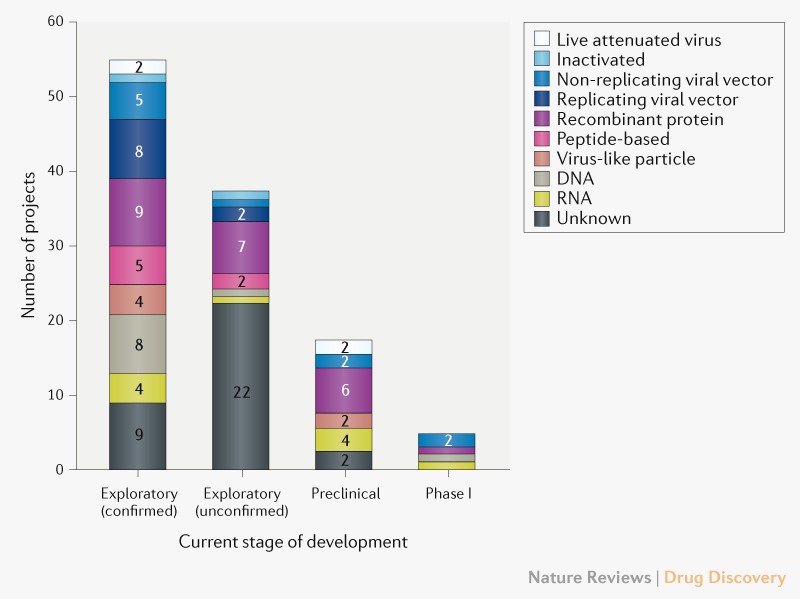
Who covid vaccine candidate. As of 23 August 2021 a total of 4619976274 vaccine doses have been administered. And 4 design and coordinate a multiple-site international randomized controlled trial the Solidarity trial for vaccines. Graduate University of Chinese Academy of Sciences SARS Pre-Clinical DNA DNA vaccine VRC-SRSDNA015-00-VP.
2 map candidate vaccines and their clinical trial worldwide publishing a frequently-updated landscape of vaccines in development. See WHOs landscape of COVID-19 vaccine candidates for the latest information on vaccines in clinical and pre-clinical development generally updated twice a week. The conduct of COVID-19 vaccine trials in the context of a candidate vaccine being issued with Emergency Use Designation raises challenging ethical questions including in relation to the use of placebo controls and unblinding of trial participants in current and future COVID-19 vaccine.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. Taiwans government on Monday approved the emergency use and production of Medigen Vaccine Biologics Corps 6547TWO COVID-19 vaccine candidate a major step in the islands plans to. Tate Reeves said Sunday that a 23-person active-duty military Covid-19 response team was deployed in Jackson to.
Nine CEPI-supported candidate vaccines are part of the COVAX initiative with a further nine candidates under evaluation and procurement conversations on-going with additional producers not currently receiving research and development RD funding through COVAX giving COVAX the largest and most diverse COVID-19 vaccine portfolio in the world. Top 10 COVID scams And that included the sharing of information and data biological samples resources technology and tools he said. Candidate vaccine Developer Coronavirus target Current stage of clinical evaluationregulatory status- Coronavirus candidate Same platform for non-Coronavirus candidates Non-Replicating Viral Vector Adenovirus Type 5 Vector CanSino Biological IncBeijing Institute of Biotechnology COVID-19 Phase 2.
In response to the Statement on the sixth meeting of the International Health Regulations 2005 Emergency Committee regarding the coronavirus disease COVID-19 pandemic and the need for WHO to support Member States to deliver COVID-19 vaccines at scale with digital tools WHO has developed this guidance and technical specifications document in collaboration with a multi-disciplinary group of experts. The Moderna COVID19 vaccine pINN. Covax the global program co-sponsored by the WHO that tries to secure vaccines for nations with less financial clout has delivered 40 million doses to.
Fake vaccines fake cremation and recycled swabs. 3 rapidly evaluate and screen for the most promising candidate vaccines simultaneously before they are tested in humans. The latest coronavirus news updated every day including coronavirus cases the latest news features and interviews from New.
Vaccine protection wanes within six months study hints. Globally as of 556pm CEST 24 August 2021 there have been 212357898 confirmed cases of COVID-19 including 4439843 deaths reported to WHO. WHOs COVID-19 dashboard updated daily also features the number of vaccine doses administered globally.
Biojector used 71 National Institute of Allergy and Infectious Diseases NIAID. ImmunityBio is developing a second-generation Covid-19 adenovirus vaccine candidate that targets both spike and nucleocapsid DNA in SARS-CoV-2. In May 2020 the firms vaccine candidate which is being manufactured by NantKwest was selected to participate in Operation Warp Speed a national program to accelerate Covid-19 vaccine development.
Boost vaccine 5253 Sun Yat-sen University China SARS Pre-Clinical DNA 3a DNA vaccine 54 State Key Laboratory of Virology. This large international randomized controlled clinical trial is designed to enable an expeditious agile and concurrent evaluation of the benefits and risks of multiple candidate preventive vaccines against COVID-19 at international sites with sufficient COVID-19 attack rates. In Mississippi where 368 of the population is fully vaccinated Gov.
It is authorized for use in people aged twelve years and older in some jurisdictions. The international TPP team was formed to 1 assess the development of the most promising candidate vaccines.
Your child cant get COVID-19 from any COVID-19 vaccine including the Pfizer-BioNTech vaccine. Both the Pfizer and the AstraZeneca COVID-19 vaccines require the full 2 dose course for the best immune response.
World Health Organization Who Vaccines Offer Strong Protection Against Covid 19 But You Must Take All The Recommended Doses Facebook
A second dose is then required to achieve the vaccines full potential.

How long for covid vaccine to offer protection. Your child will need a second shot of the Pfizer-BioNTech COVID-19 Vaccine 3 weeks after their first shot. That protection can last for at least two months according to Vinh. The Moderna and Pfizer-BioNTech vaccines offer immunity against COVID-19 for at least six months and might offer protection for up to two to three years.
Pfizer chief sees need. The Pfizer-BioNTech COVID-19 vaccine is 95 effective in preventing the COVID-19 virus with symptoms in people age 16 and older. Its going to be somewhere in the.
The Pfizer-BioNtech and Moderna COVID-19 vaccines will likely provide protection against the coronavirus for years if it doesnt evolve significantly a small new study suggests. While the current COVID-19 vaccines will likely last for at least about a year they probably will not offer lifelong protection as with measles shots said Dr Kathleen Neuzil a vaccine expert at the University of Maryland. AstraZeneca Covid vaccine gives powerful protection that may last a lifetime study finds.
The data also showed that a second dose given 21 days. But more data on the mRNA vaccines is emerging. Compared to data from 16-22 July from the Centers for Disease Control and Prevention CDC demand for covid-19 vaccines is up 70 percent in.
Individuals may not have the best protection until 7-14days after their second dose of the vaccine. Clinical trials show COVID vaccine protection is optimal from about two weeks after your second dose. Your child may get a COVID-19 vaccine and other vaccines at the same visit or without waiting 14 days between vaccines.
Recovering from COVID-19 is believed to offer some protection against the virus but its unclear how long that protection lasts and its possible to get COVID-19 more than once. How long does it take for the COVID-19 vaccine to work. One is that the three coronavirus vaccines authorized for use in the United States provide a high degree of protection for at least three months based on clinical trials that began as early as last July.
However they will most likely have to be administered annually. The Johnson Johnson Moderna and Pfizer-BioNTech vaccines will likely protect against current variants of COVID-19. The vaccine is 100 effective in preventing the COVID-19 virus in children ages 12 through 15.
Regardless of which vaccine you get you wont reach full protection until two weeks after your second or final dose. This vaccine is for people age 12 and older. But after that the vaccinated infection rate dropped.
During the first two weeks after the first dose recipients were almost as likely as control subjects given a placebo to develop COVID-19. Studies show that getting one dose of a COVID-19 vaccine offers more than 70 per cent protection but the second dose is what helps fully strengthen the. US preparing for 1-year COVID-19 booster shots.
The Pfizer-BioNTech vaccine researchers concluded offered very good protection against the Delta variant and demonstrated 79 percent effectiveness 14. Nearly completely protect against severe disease and death in. Thats about how long it takes your immune system to mount an antibody response to the vaccine.
Popular Posts
-
Also known as the malar rash the butterfly rash is actually one of several skin concerns associated with lupus. These rashes are mostly foun...
-
Seek care immediately if you experience this symptom. What are the symptoms of penicillin allergy. Penicillin Allergy Often Inaccurately R...
-
Moreover a person having hives or scabies could also have rash in most cases. It may cause burning or stinging sensation. Are Hives And Ra...
Featured Post
best fish to feed cats
Cats And Fish: What Fish Is Best To Feed Your Cat? . WebThe best types of fish to feed your cat include salmon, tuna, and cod. These f...

ads


