The Guidance aims to support WHO. Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA.
Tracking Covid 19 Vaccines And Therapeutics Mckinsey
But its not vaccines that will stop the pandemic its vaccination.
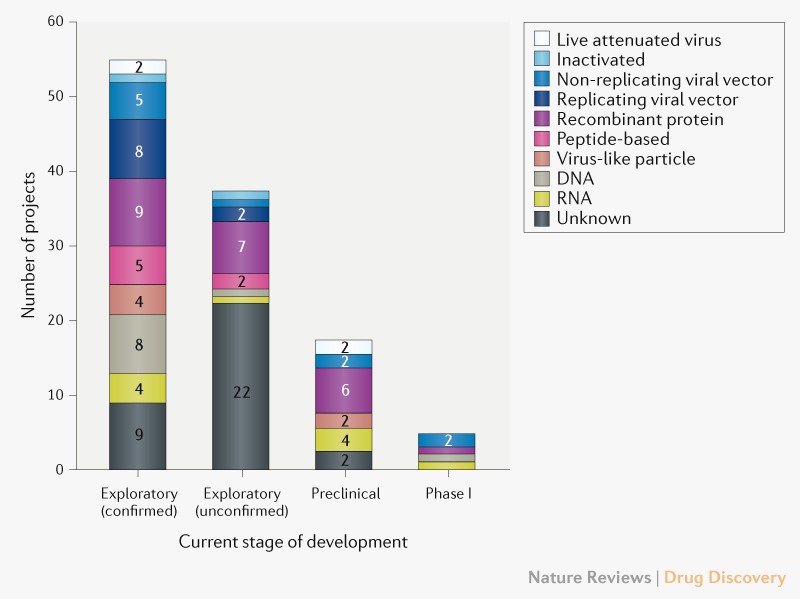
Who covid vaccine candidate. As of 23 August 2021 a total of 4619976274 vaccine doses have been administered. And 4 design and coordinate a multiple-site international randomized controlled trial the Solidarity trial for vaccines. Graduate University of Chinese Academy of Sciences SARS Pre-Clinical DNA DNA vaccine VRC-SRSDNA015-00-VP.
2 map candidate vaccines and their clinical trial worldwide publishing a frequently-updated landscape of vaccines in development. See WHOs landscape of COVID-19 vaccine candidates for the latest information on vaccines in clinical and pre-clinical development generally updated twice a week. The conduct of COVID-19 vaccine trials in the context of a candidate vaccine being issued with Emergency Use Designation raises challenging ethical questions including in relation to the use of placebo controls and unblinding of trial participants in current and future COVID-19 vaccine.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. Taiwans government on Monday approved the emergency use and production of Medigen Vaccine Biologics Corps 6547TWO COVID-19 vaccine candidate a major step in the islands plans to. Tate Reeves said Sunday that a 23-person active-duty military Covid-19 response team was deployed in Jackson to.
Nine CEPI-supported candidate vaccines are part of the COVAX initiative with a further nine candidates under evaluation and procurement conversations on-going with additional producers not currently receiving research and development RD funding through COVAX giving COVAX the largest and most diverse COVID-19 vaccine portfolio in the world. Top 10 COVID scams And that included the sharing of information and data biological samples resources technology and tools he said. Candidate vaccine Developer Coronavirus target Current stage of clinical evaluationregulatory status- Coronavirus candidate Same platform for non-Coronavirus candidates Non-Replicating Viral Vector Adenovirus Type 5 Vector CanSino Biological IncBeijing Institute of Biotechnology COVID-19 Phase 2.
In response to the Statement on the sixth meeting of the International Health Regulations 2005 Emergency Committee regarding the coronavirus disease COVID-19 pandemic and the need for WHO to support Member States to deliver COVID-19 vaccines at scale with digital tools WHO has developed this guidance and technical specifications document in collaboration with a multi-disciplinary group of experts. The Moderna COVID19 vaccine pINN. Covax the global program co-sponsored by the WHO that tries to secure vaccines for nations with less financial clout has delivered 40 million doses to.
Fake vaccines fake cremation and recycled swabs. 3 rapidly evaluate and screen for the most promising candidate vaccines simultaneously before they are tested in humans. The latest coronavirus news updated every day including coronavirus cases the latest news features and interviews from New.
Vaccine protection wanes within six months study hints. Globally as of 556pm CEST 24 August 2021 there have been 212357898 confirmed cases of COVID-19 including 4439843 deaths reported to WHO. WHOs COVID-19 dashboard updated daily also features the number of vaccine doses administered globally.
Biojector used 71 National Institute of Allergy and Infectious Diseases NIAID. ImmunityBio is developing a second-generation Covid-19 adenovirus vaccine candidate that targets both spike and nucleocapsid DNA in SARS-CoV-2. In May 2020 the firms vaccine candidate which is being manufactured by NantKwest was selected to participate in Operation Warp Speed a national program to accelerate Covid-19 vaccine development.
Boost vaccine 5253 Sun Yat-sen University China SARS Pre-Clinical DNA 3a DNA vaccine 54 State Key Laboratory of Virology. This large international randomized controlled clinical trial is designed to enable an expeditious agile and concurrent evaluation of the benefits and risks of multiple candidate preventive vaccines against COVID-19 at international sites with sufficient COVID-19 attack rates. In Mississippi where 368 of the population is fully vaccinated Gov.
It is authorized for use in people aged twelve years and older in some jurisdictions. The international TPP team was formed to 1 assess the development of the most promising candidate vaccines.
Popular Posts
-
Also known as the malar rash the butterfly rash is actually one of several skin concerns associated with lupus. These rashes are mostly foun...
-
Seek care immediately if you experience this symptom. What are the symptoms of penicillin allergy. Penicillin Allergy Often Inaccurately R...
-
Moreover a person having hives or scabies could also have rash in most cases. It may cause burning or stinging sensation. Are Hives And Ra...
Featured Post
best fish to feed cats
Cats And Fish: What Fish Is Best To Feed Your Cat? . WebThe best types of fish to feed your cat include salmon, tuna, and cod. These f...

ads

